These images are best appreciated in stereo, because in most views the combination of 3D geometry and surface coloration makes the structure almost impossible to figure out from a single view.
But let's start with a view that is simple enough to work even as a single image.
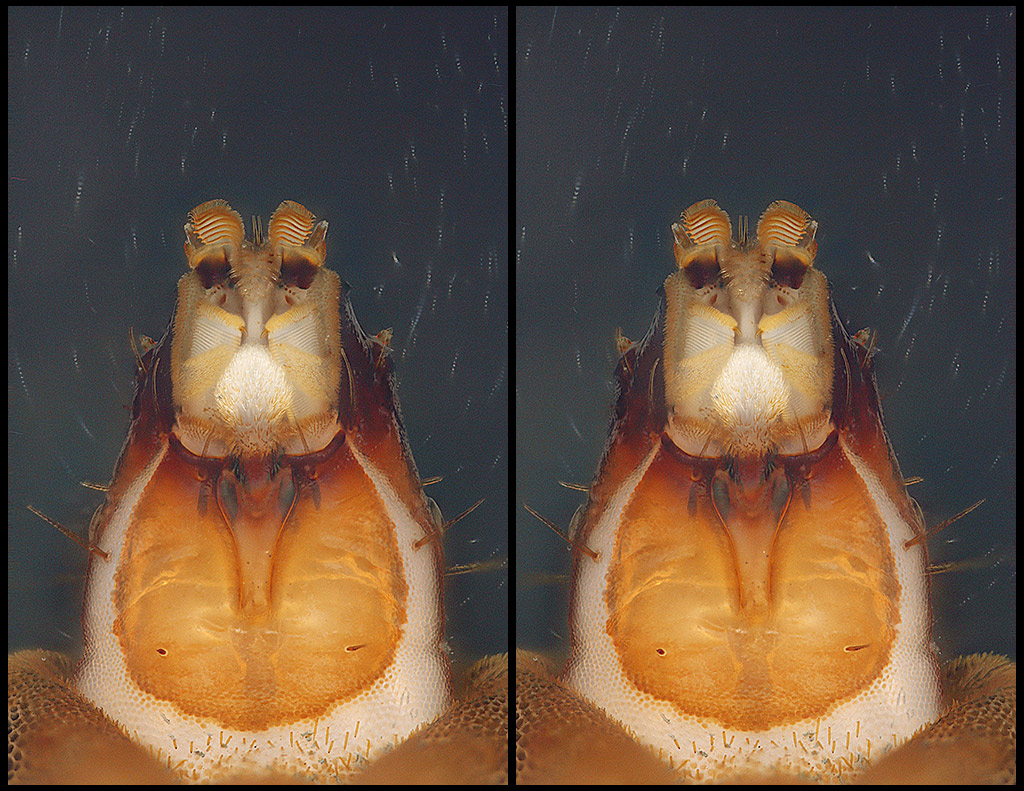
This is a slightly oblique ventral pose ("underside, looking up"), showing the entire head. This is almost a whole APS-C frame shot at 10X magnification, so a little over 2 mm from bottom to top.
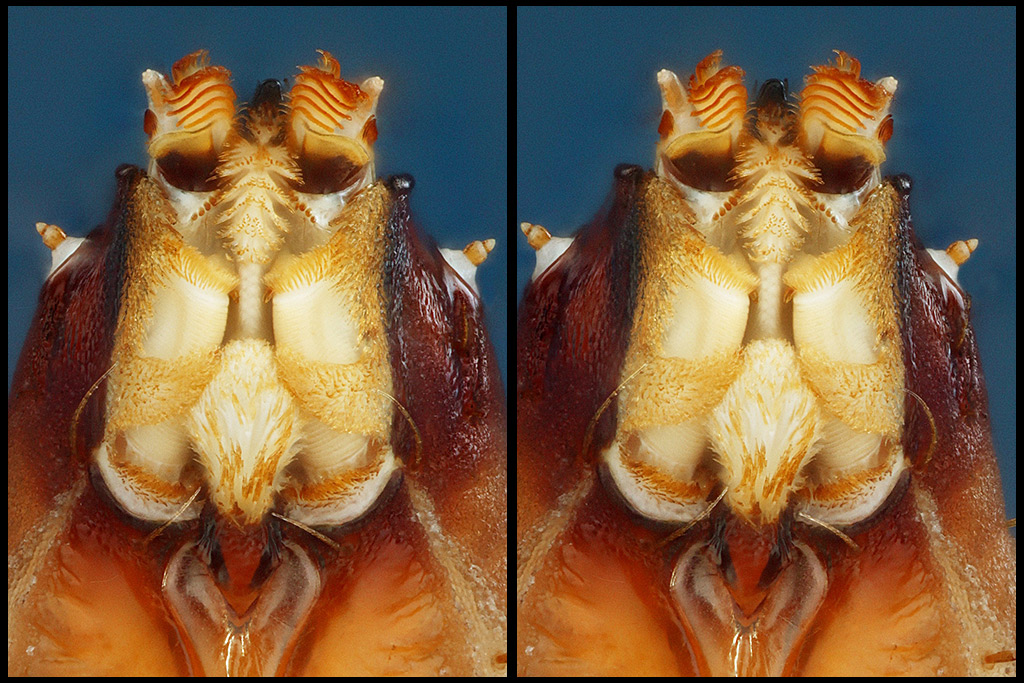
Following is a closer crop of the same image.

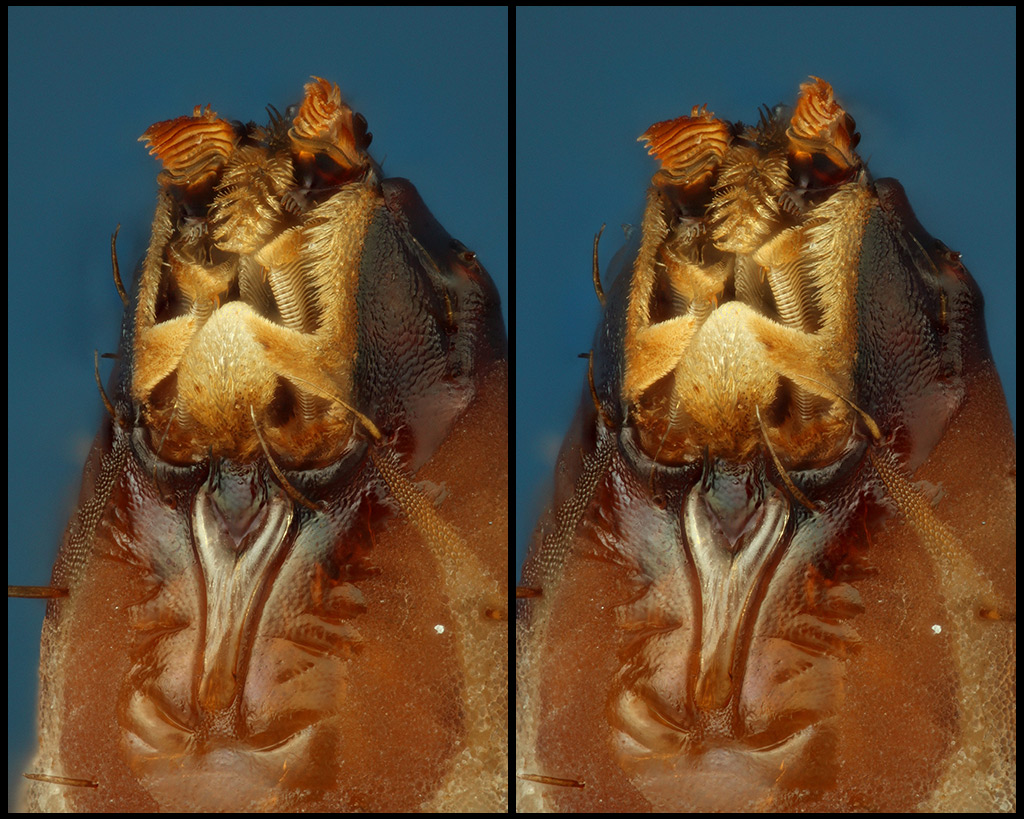
Now, the dorsal view ("above, looking down").

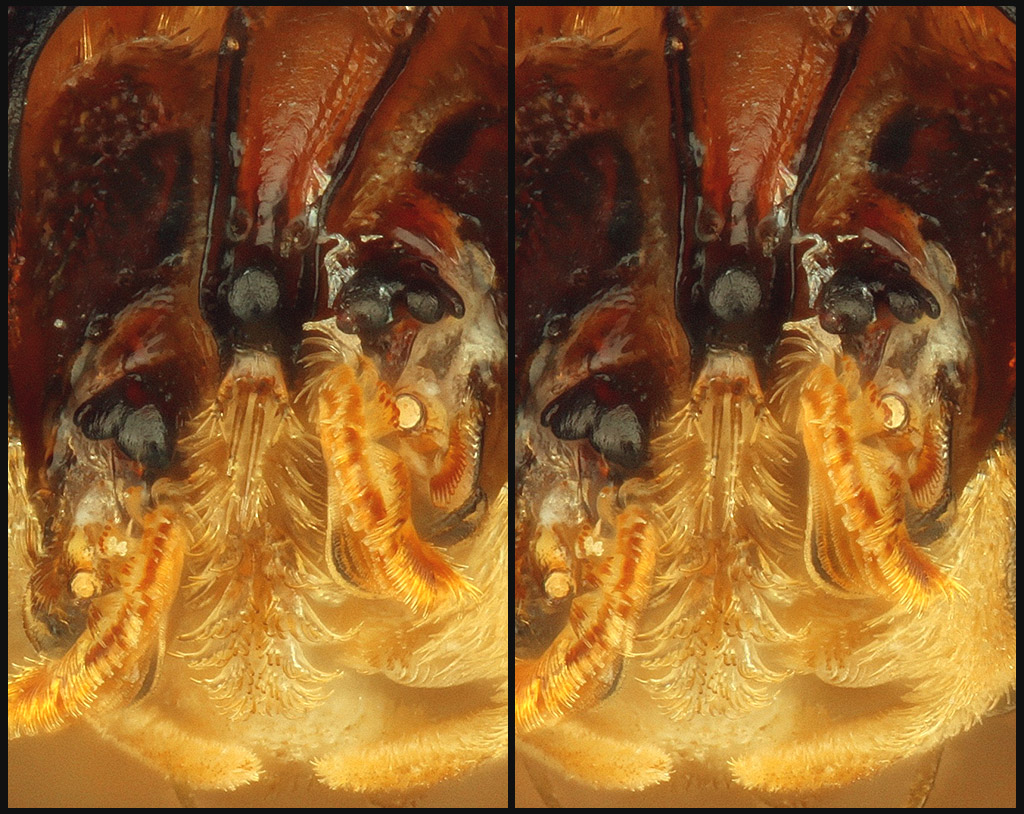
Following, a closer crop.

Finally, here is the frontal view, looking into the face. The two "eyes" are actually antennae in their sockets.
The technical paper that I mentioned is "An in-depth description of head morphology and mouthparts in larvae of the black soldier fly Hermetia illucens", Daniele Bruno et.al, Arthropod Structure & Development 58 (2020) 100969, currently available at https://www.entomofago.eu/wp-content/up ... hparts.pdf . My photos here can be compared with the paper's SEM images in Figures 2A and 2B, but note that their specimen shown in 2B captures the mandibles in a position that is pulled down much farther across the epipharynx than the single specimen that I'm showing here.
I was blown away by the complexity of these mouthparts. I have not spent much time looking at fly larvae, and the only kind that I've looked at in detail seemed much simpler (HERE). But that other kind was a cherry fruit fly, whose larvae live in a very simple and stable environment: the pulp of a cherry. In contrast, black soldier fly larvae are general purpose feeders that live on a wide range of decaying plant matter. Considering their diet, it makes perfect sense that the BSFL's would be equipped with a complicated mechanism that is capable of grinding and separating a wide variety of food textures. The paper by Bruno et.al. discusses the anatomy and presumed mode of operation in great detail, much more than can be seen in my photos. Quoting part of their summary:
Also new to me was the method that I used to prepare this specimen. Many of the structures, particularly the delicate tree-like branching of the epipharynx, do not hold up well to simple drying in alcohol. So instead, I adapted the method that the paper describes for preparing their SEM specimens: boiling in water for a few seconds, fixation of the proteins by glutaraldehyde in a neutral buffered solution, followed by dehydration in alcohol plus washing in hexamethyldisilazane (HMDS) and drying in air as a substitute for critical point drying. I'll post separately about that process.The head walls are extremely robust and the conical shape makes it easy to penetrate the food substrate thanks to the pressure exerted by the entire larval body. The mouthparts resemble a sort of tunnel boring machine, like that used in modern times, but instead of a “whole section” disc grinder, the mouthparts move on a vertical plane, that of the mandibular-maxillary apparatus. According to the morphological evidence presented herein,we hypothesize that these two appendages rotate from a horizontal rest position onwards and downwards until the mandibular hooks touch and trespass the ventral membranous lobes, describing an angle of about 100/110°. In this way the hooks grasp more or less coarse particles of the semiliquid food and the finer rasp of the stipes grinds them into smaller parts, with the help of the lacinial teeth. The food is probably driven towards the mouth, and the hypopharynx and the chitinous septum above separate the finer particles from the coarse and inorganic particles, the last ones being expelled ventrally, probably also by contractions of the hypopharynx and the prementum. The “good” particles enter the pharynx and reach the “grinding mill”, where they undergo the last mechanical treatment. The advancement into the food substrate is likely supported by the sensorial equipment of the epipharyngeal (labral) comb of sensorial appendages, which surround the hypopharynx anteriorly.
...corroborates the hypothesis of a multifunctional apparatus that easily adapts to ingesting semiliquid food derived from any possible decaying organic matter. The exact function of the epipharynx and the hypopharynx are still somewhat unclear and in vivo video recordings could perhaps give more insights into the exact role of all the mouthparts as a whole.
The photographic process here was routine: specimen mounted on an insect pin with a bit of Bondic, illumination diffused through a plastic hemisphere painted white, Mitutoyo M Plan Apo 10X NA 0.28 with a Raynox DCR-150 tube lens, Canon T1i at ISO 100, second-curtain sync flash with 0.5 second shutter speed with room lights off, focus-stepped at 0.005 mm, Zerene Stacker synthetic stereo at 4 degrees off axis (8 degrees total separation).
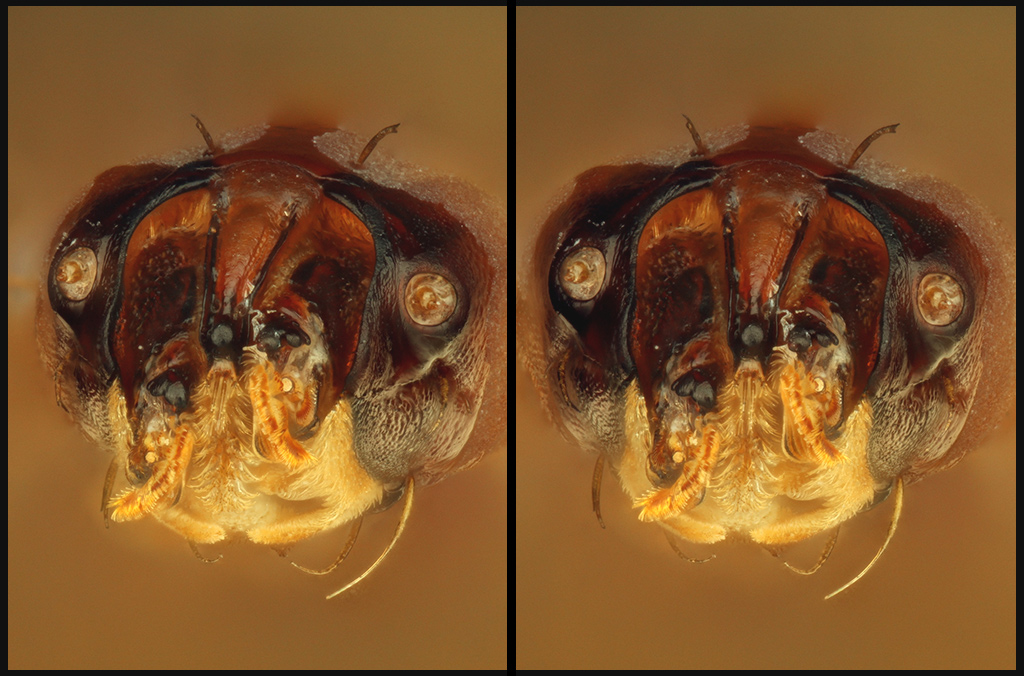
The colors shown above do not accurately reflect what I see by eye. By eye, as seen through a dissecting scope with LED illumination, the specimen appears to have much more muted colors, with none of "glowing orange" effect in the galea and other light-colored areas. The following crop from a cell phone camera, shot through a 45X dissecting scope with Jansjö LED illumination, is more realistic:

For the high resolution stacked images, the camera and illumination are properly color balanced for neutral gray. So, I expect what's going on is a form of metamerism like I described at viewtopic.php?t=37664 ("interesting aspect #3"). That is, I expect that light from the chitin is enhanced in some IR or near IR component that is captured by the camera as red instead of missed by eye. But rather than adjust the colors to give a more realistic appearance, I decided to leave them as captured.
I hope you find at least some of this interesting, though I don't expect anybody else to be as fascinated as I am!
--Rik